Our Research:
The human genome is infested with hundreds of thousands of sequences called retrotransposons. Retrotransposons can self-replicate and insert their sequence semi-randomly back into the genome to generate mutations. In humans, an element called Long Interspersed Nuclear Element-1 (LINE-1, or L1) is the current dominant retrotransposon. L1 controls essentially all transposition in humans.
Simplified depicition of L1 replication cycle:1) transcription of L1 mRNA and export to cytoplasm 2) translation of ORF1/ORF2 and formation of L1 RNP 3) traffic to nucleus by unknown mechanism 4) reverse transcription/integrationTo replicate, L1 mRNA and two L1-encoded proteins (called ORF1p and ORF2p) form a cytoplasmic ribonucleoprotein particle (RNP) intermediate. The L1 RNP enters the nucleus and reverse transcribes L1 mRNA at new chromosomal sites (see the figure). This process, called retrotransposition, has been spectacularly successful in humans and has generated over one-third of the human genome. In addition, expression of L1 RNPs in humans and/or model organisms is associated with mutations, DNA damage, aging, and various disorders, such as cancer, infertility, and neurologic disease. In most of these cases we do not know whether L1 is simply correlated with these disorders, or if L1 plays a causative role in these disorders. Our inability to convincingly address this question is due to a gap in our basic understanding of LINE biology, which limits our ability to manipulate endogenous L1 activity in a specific manner.
Cell and Molecular Biology of L1
We use a combination of genetic, molecular, cell biological and computational approaches to understand how L1 replicates. We are particularly interested in how L1 exploits cell host Cytoplasmic sequestration of L1 RNPs:Confocal image of HeLa cells expressiing L1. Green=L1 ORF1p.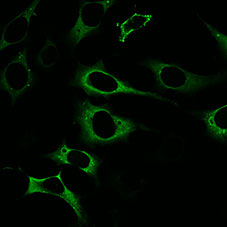 proteins to facilitate retrotransposition, how L1 RNPs traffic around the cell, and how L1 RNPs enter the nucleus. A genetic screen performed in our lab revealed that ESCRT, a complex involved in membrane budding, plays an important role for successful LINE retrotransposition. We are currently investigating how ESCRT is utilized by L1 RNPs. We speculate that ESCRT allows L1 RNPs pass through membranes as they traffic to the nucleus. In addition, although we know L1 RNPs must access the nucleus to integrate, nuclear entry appears to be inefficient in wild-type cells, as most L1 RNPs are sequestered in the cytoplasm. We are using genetics to identify how the cell is able to recognize L1 RNPs and keep them out of the nucleus.
proteins to facilitate retrotransposition, how L1 RNPs traffic around the cell, and how L1 RNPs enter the nucleus. A genetic screen performed in our lab revealed that ESCRT, a complex involved in membrane budding, plays an important role for successful LINE retrotransposition. We are currently investigating how ESCRT is utilized by L1 RNPs. We speculate that ESCRT allows L1 RNPs pass through membranes as they traffic to the nucleus. In addition, although we know L1 RNPs must access the nucleus to integrate, nuclear entry appears to be inefficient in wild-type cells, as most L1 RNPs are sequestered in the cytoplasm. We are using genetics to identify how the cell is able to recognize L1 RNPs and keep them out of the nucleus.
L1-host interactions in the germ line
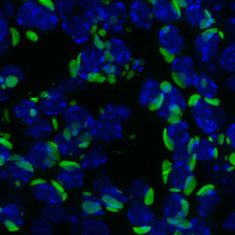 L1 ORF1 expression in mouse testes:Confocal image of endogenous L1 in the seminiferous tublues of mouse testes. Green=L1 ORF1p. Blue=DAPI (nuceli)
L1 ORF1 expression in mouse testes:Confocal image of endogenous L1 in the seminiferous tublues of mouse testes. Green=L1 ORF1p. Blue=DAPI (nuceli)
Evolutionarily successful transposons should be active in the germ line, because these cells give rise to the next generation. In contrast, somatic transposition events are evolutionarily non-productive, as these events are lost when the host organism dies. Consistent with this expectation, transposons tend to be expressed in germ cells. We hypothesize that the co-evolution of LINEs with the germ line has led to interactions between LINE RNPs and germ line-specific proteins. Our lab is interested in identifying these interactions and determining what happens when these interactions are disrupted.
L1 dysregulation and human disease
Expression of L1 leads to double stranded DNA breaks and mutations. As a consequence, it is no surprise that our cells have evolved mechanisms to limit L1 retrotransposition to relatively low levels in vivo. However, what happens when these mechanisms fail? Numerous studies have shown correlation between L1 overexpression and disease, such as neurological disorders, aging, cancer and infertility. Using mouse models, we are asking whether L1 dysregulation contributes to cancer or infertility in mammals. To this end, our discovery of host factors important for L1 retrotransposition allows us to reduce endogenous L1 activity in vivo. In addition, we are performing various screens to identify small molecules to directly block toxic enzymatic activities of L1 RNPs.
Han Laboratory
Tulane Cancer Center
1700 Tulane Avenue
LCRC Room 743
New Orleans, LA 70112